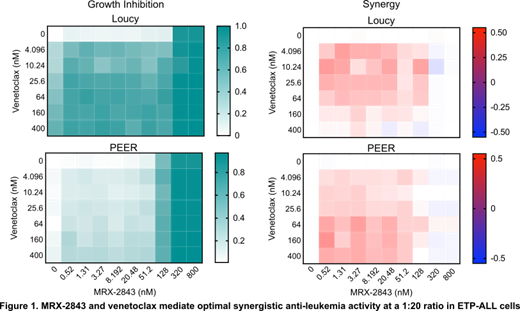Abstract
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) accounts for 15% of childhood ALL and is associated with inferior outcomes relative to B-cell ALL. Early T-precursor ALL (ETP-ALL) is a subset of T-ALL characterized by an immature T cell phenotype, resistance to therapy, and high rates of induction failure. MERTK receptor tyrosine kinase is ectopically expressed in 40-50% of T-ALLs, particularly those with an immature T cell phenotype, suggesting a role in ETP-ALL. Inhibition of MERTK using shRNA delayed leukemia progression and prolonged survival in a T-ALL xenograft model, implicating MERTK as a therapeutic target. MRX-2843 is an orally available dual MERTK/FLT3 inhibitor currently in phase I clinical trials. The anti-apoptotic protein B-cell lymphoma-2 (BCL-2) is specifically expressed in immature T cell precursors, is preferentially expressed in ETP-ALL compared to other T-ALLs, is essential for ETP-ALL cell survival, and is regulated downstream of MERTK in acute leukemia cells. Thus, combination therapies targeting these two proteins may be particularly effective to treat ETP-ALL.
Methods
Loucy and PEER ETP-ALL cell lines were cultured with vehicle or MRX-2843. Phosphorylated and total MERTK were assessed by immunoblot. Relative cell numbers were measured using Presto Blue reagent. Cells were stained with PoPro-1-iodide and propidium iodide and apoptotic and dead cells were quantitated by flow cytometry. T-ALL patient samples were cultured with UNC2025, a close analogue of MRX-2843, and relative cell numbers were assessed using MTS reagent. Orthotopic xenografts were established in NSG or NSGS mice using luciferase-expressing Jurkat cells (T-ALL), luciferase-expressing Loucy cells (ETP-ALL) or an ETP-ALL patient sample and leukemia burden was monitored by bioluminescence imaging or flow cytometry. MRX-2843 (65 mg/kg or 75 mg/kg) or saline vehicle were administered orally once daily. Differences in disease burden were assessed with the Mann-Whitney-U test or one-way ANOVA. Survival was determined by Kaplan-Meier analysis. Loucy and PEER cells were plated and screened in quadruplicate against >150 pairwise combinations of MRX-2843 and the BCL-2 inhibitor venetoclax in a high-throughput format. Synergy was calculated using the response additivity model.
Results
Treatment with MRX-2843 mediated a dose-dependent decrease in phosphorylated MERTK, inhibited expansion of ETP-ALL cells, and induced cell death in vitro. Fifty-four percent (21/39) of primary T-ALL patient samples were sensitive to UNC2025 with an IC 50≤550 nM, including 2/5 (40%) pediatric samples and 10/19 (53%) adolescent/young adult samples. Treatment with MRX-2843 significantly reduced leukemia burden in cell line-derived T-ALL and ETP-ALL xenograft models and prolonged survival by 50% and 13% in the T-ALL (n=10, p<0.0001) and ETP-ALL (n=10, p=0.0136) models, respectively. Similarly, in a patient-derived ETP-ALL xenograft model, treatment with MRX-2843 reduced peripheral blood disease burden by 83% and spleen weight by 64% compared to vehicle-treated mice (n=8, p<0.001) and prolonged survival by 41% (n=8, p=0.0016). MRX-2843 mediated anti-leukemia activity in combination with venetoclax and a dose ratio of 1:20 MRX-2843:venetoclax provided optimal synergy in Loucy and PEER ETP-ALL cells in vitro (Figure 1).
Conclusions
MRX-2843 has therapeutic activity in ETP-ALL cell culture and xenograft models and over half of T-ALL patient samples were sensitive to MERTK/FLT3 inhibition. MRX-2843 also mediated synergistic anti-leukemia activity against ETP-ALL cells in combination with venetoclax, with an optimal molar ratio of 1:20. These data demonstrate the therapeutic potential of MRX-2843 in patients with T-ALL, suggest that MRX-2843 may be particularly active alone and in combination with venetoclax in the ETP-ALL subset, and provide rationale for clinical testing of MRX-2843, with the ultimate goal to progress to trials evaluating MRX-2843 in combination with other agents. Toward this end, MRX-2843 monotherapy will be tested in patients with relapsed leukemia in an upcoming clinical trial (NCT04872478).
Wang: Meryx: Other: Equity ownership; University of North Carolina: Patents & Royalties. Frye: University of North Carolina: Patents & Royalties; Meryx: Membership on an entity's Board of Directors or advisory committees, Other: Equity ownership. Earp: Meryx: Membership on an entity's Board of Directors or advisory committees, Other: Equity ownership. Tyner: Petra: Research Funding; Incyte: Research Funding; Takeda: Research Funding; Janssen: Research Funding; Astrazeneca: Research Funding; Array: Research Funding; Constellation: Research Funding; Seattle Genetics: Research Funding; Schrodinger: Research Funding; Genentech: Research Funding; Gilead: Research Funding; Agios: Research Funding. DeRyckere: Meryx: Other: Equity ownership. Graham: Meryx: Membership on an entity's Board of Directors or advisory committees, Other: Equity ownership.